distinguish between ketone and aldehyde Aldehyde ketone distinguish chemistrypage
Have you ever wondered about the difference between aldehydes and ketones? These two organic compounds have very similar structures, but there are some key distinctions between them that can affect their properties and reactivity. First, let’s take a look at their structures. Both aldehydes and ketones contain a carbonyl group, which consists of a carbon atom double-bonded to an oxygen atom. In an aldehyde, the carbonyl group is located at the end of a carbon chain, while in a ketone, it is located in the middle of the chain. This difference affects the way the compounds behave in certain reactions. One of the main differences between aldehydes and ketones is their reactivity with nucleophiles. Nucleophiles are atoms or molecules that have a negative charge or a lone pair of electrons, and are able to attack the carbonyl carbon in a reaction. Aldehydes are generally more reactive than ketones towards nucleophiles, due to the fact that the carbonyl carbon in an aldehyde is more electrophilic (electron-loving) than in a ketone. This means that nucleophiles are more likely to attack the carbonyl carbon in an aldehyde than in a ketone. Another important difference between aldehydes and ketones is their physical properties. Aldehydes tend to have lower boiling points than ketones, due to the fact that they have a more polarized carbonyl group. This means that the intermolecular forces between aldehyde molecules are weaker than between ketone molecules, and so less energy is required to break the bonds between them. Aldehydes also tend to be more soluble in water than ketones, again due to their more polarized carbonyl group. So what are some examples of aldehydes and ketones? Some common aldehydes include formaldehyde, acetaldehyde, and benzaldehyde. Formaldehyde is a common disinfectant and preservative, while acetaldehyde is produced by the body as a result of alcohol metabolism. Benzaldehyde is responsible for the characteristic almond aroma in many foods and fragrances. Some common ketones include acetone, which is used as a solvent and a nail polish remover, and cyclohexanone, which is used in the production of nylon. In conclusion, aldehydes and ketones may have very similar structures, but their properties and reactivity can be quite different. Understanding these differences can be important in fields such as organic chemistry, where understanding the reactivity of different functional groups is crucial to designing new molecules and materials.
If you are searching about Pin by Flow - Wellness and Training on Quick Saves in 2021 | Organic you’ve came to the right web. We have 5 Pictures about Pin by Flow - Wellness and Training on Quick Saves in 2021 | Organic like How will you distinguish between aldehyde and ketone?, Difference Between Aldehyde and Ketone | Structure, Properties, Naming and also Difference Between Aldehyde and Ketone – CueRead. Here it is:
Pin By Flow - Wellness And Training On Quick Saves In 2021 | Organic
 www.pinterest.com.mxAldehydes And Ketones: The Carbonyl Functional Group, Naming, Reactions
www.pinterest.com.mxAldehydes And Ketones: The Carbonyl Functional Group, Naming, Reactions
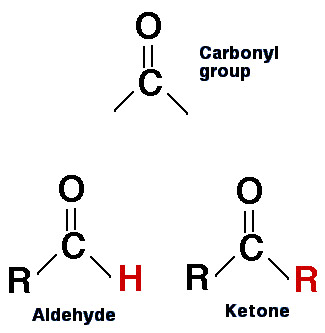 biology.reachingfordreams.comketone ketones aldehydes carbonyl group aldehyde functional structure difference between carboxylic compound molecule acids biology groups chemistry two reactions diagram
biology.reachingfordreams.comketone ketones aldehydes carbonyl group aldehyde functional structure difference between carboxylic compound molecule acids biology groups chemistry two reactions diagram
Difference Between Aldehyde And Ketone – CueRead
 www.cueread.comDifference Between Aldehyde And Ketone | Structure, Properties, Naming
www.cueread.comDifference Between Aldehyde And Ketone | Structure, Properties, Naming
 in.pinterest.comaldehyde ketone ketones pediaa aldehydes keton aldehyd carboxylic unterschied
in.pinterest.comaldehyde ketone ketones pediaa aldehydes keton aldehyd carboxylic unterschied
How Will You Distinguish Between Aldehyde And Ketone?
 chemistrypage.inaldehyde ketone distinguish chemistrypage
chemistrypage.inaldehyde ketone distinguish chemistrypage
How will you distinguish between aldehyde and ketone?. Aldehyde ketone ketones pediaa aldehydes keton aldehyd carboxylic unterschied. Aldehydes and ketones: the carbonyl functional group, naming, reactions